This feature is available to administrators that have the "Study type management" permission.
Study types can be defined within the OnePacs system, such that interpreted cases can be classified and billed for, or reimbursed, appropriately.
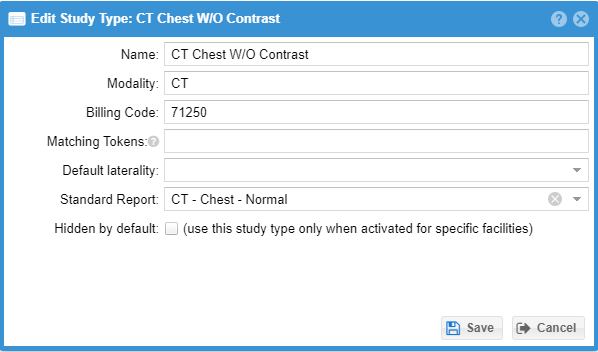
When a study type is created, a global name for the study type is defined. Study types can be associated with a study by any of the following mechanisms:
- automatically via post-processing rules
- selected by a technician or radiology assistant when confirming the study
- selected by the radiologist when completing the report.
According to how the facility is configured, this step may be required, optional, or not available.
The global billing name should be the most general name applicable to the study in question across all serviced facilities, such as “CT Abdomen - Three phase liver”.
Extra matching tokens can be entered optionally to match various fields like study description to highlight certain study types for quicker selection.
Shared standard report texts can optionally be associated with a study type. This can enter the associated standard report text if the study type is selected for the study either during confirmation or when the radiologist is completing the report.
A study type may be hidden by default for special cases.
Related Pages