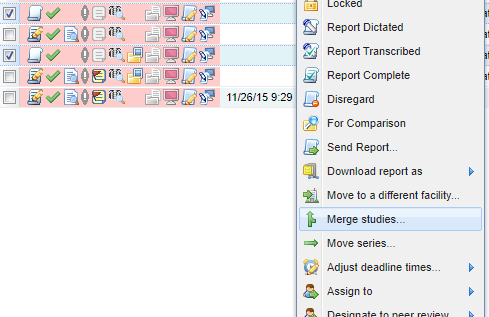
At times it may be necessary to modify study data after transmission of a study to OnePacs. For example, sometimes a paperwork process uses a different study identifier (Study Instance UID) and it results in two different studies. These studies can be merged.
Requirements:
- The user must have the “Modify patient/study/series data” permission
- The facility must be configured to allow such modifications.
- Studies are not reported
If you are unable to merge studies for your organization check these two settings or contact the administrator for your group.
You will receive a warning if the studies have different modalities.
If you are unable to move studies for your organization check these two settings or contact the administrator for your group.
Find the studies on the worklist and select the two studies to be merged. Click the "Manage" Button and the "Merge studies..." option.
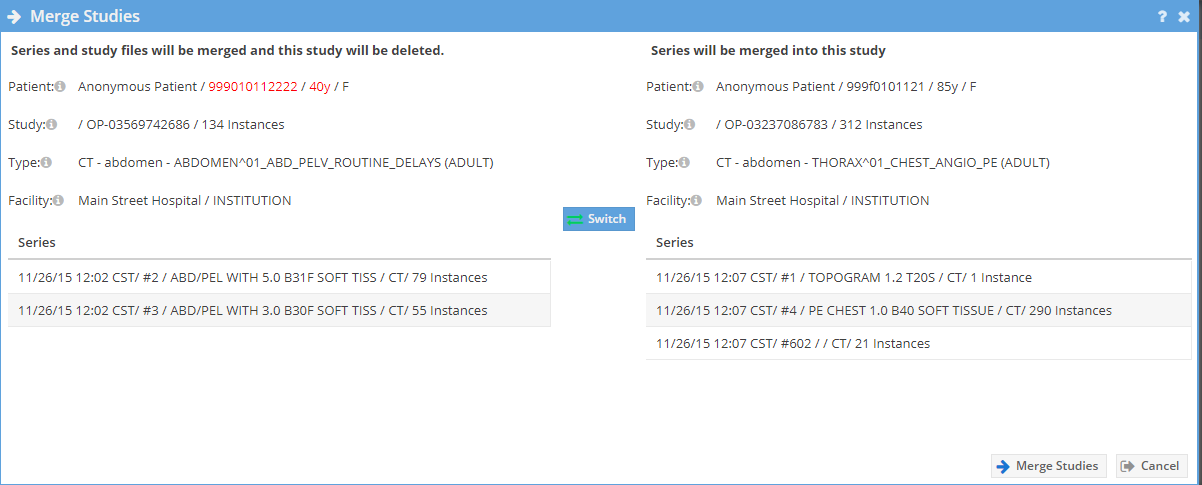
A new window will open. If the patient information is different it is highlighted. Caution should be exercised when merging studies. Click the "Merge Studies" button to merge the studies.