Moving One or More Studies to a Different Facility
It may be necessary to move a study from one facility to another within your organization. Such a scenario may occur if a study was uploaded via the web interface to the wrong facility or DICOM institution based routing failed because of an incorrect DICOM header value.
Requirements
Selecting a study to move
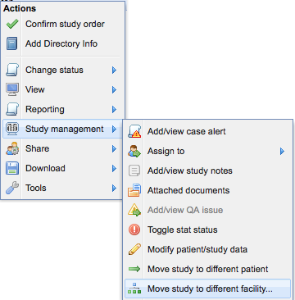
Studies may be moved individually or in bulk. To move an individual study, right-click on the study on the worklist and select "Study management -> Move study to a different facility"
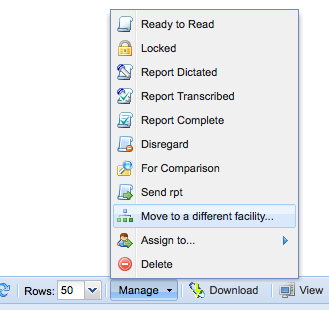
To move multiple studies, select multiple studies on the worklist and use the "Manage" menu in the bottom toolbar to select "Move to a different facility".
Selecting a destination facility
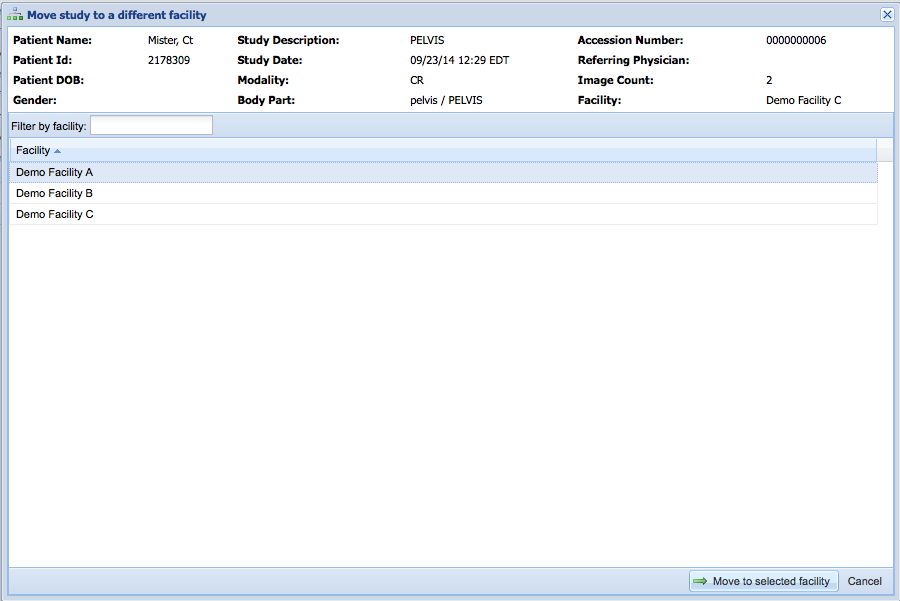
Next, you will prompted to select a destination facility. Select a facility from the list of available facilities.
Click on "Move to facility" to initiate the move.
Executing the move
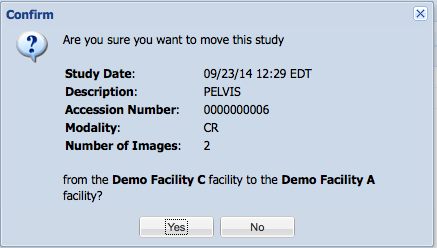
You will be prompted to confirm the study move prior to it being performed.
Upon clicking yes, the study will be moved to the new facility.